Abstract
Background:
Transfusional iron overload is a poor prognostic factor in patients undergoing hematopoietic stem cell transplantation (HSCT). In multiple clinical studies, elevated serum ferritin correlated with inferior transplant outcomes; however, ferritin is an imperfect marker of iron burden and may not correlate with other markers, such as liver iron content. These studies are also confounded because increased transfusional need often reflects more severe disease, an independent predictor of worse outcome. Thus, it remains unclear whether the correlation between iron status and transplant outcome is due to transfusional iron overload alone, or is more reflective of other comorbidities. We developed a mouse model of parenteral iron overload followed by high dose chemotherapy and allogeneic or syngeneic HSCT to study, in isolation, the effects of iron overload on engraftment and survival after transplant.
Methods:
Cohorts of female C57BL/6 (H-2Kb) recipients received 0, 5, 25, or 50mg iron dextran, in divided doses over 2 weeks. Mice were then conditioned with busulfan (20mg/kg daily x 5 days) and cyclophosphamide (100mg/kg daily for the last 3 days). Two days later, mice received 30 x 106 bone marrow cells from female BALB/c (H-2Kd) donors (allogeneic HSCT) or 5 x 106 bone marrow cells from female UBC-GFP (H-2Kb) donors (syngeneic HSCT). Mice received enrofloxacin (10mg/kg daily x 5 days) after transplant. Engraftment was evaluated by flow cytometry as the proportion of donor cells in blood or bone marrow. Iron-related parameters were measured at the time of euthanasia (Day +28 post-transplant). Statistical analysis was performed with GraphPad Prism.
Results:
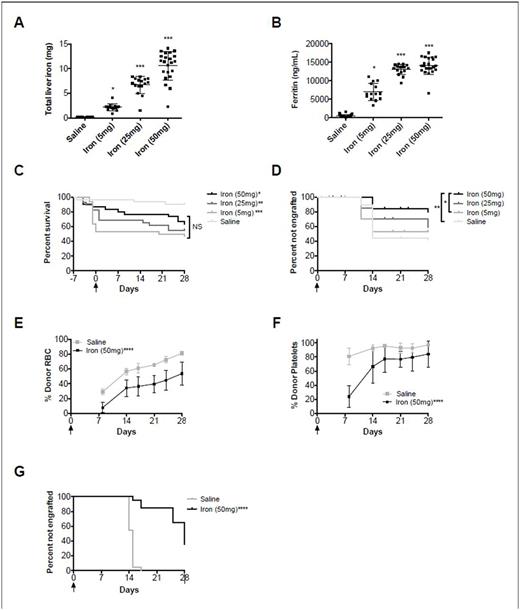
Our model of iron overload using iron dextran at escalating doses showed a dose-dependent increase in markers of iron load, such as total liver iron (R2=0.86) and ferritin (R2=0.84)(Fig. 1A-B; both P<0.0001 for linear trend). After allogeneic HSCT, there was a statistically significant decrease in overall survival in all groups receiving iron dextran (range: 47-63%), as compared to the saline control (90%)(Fig 1C; P<0.05); this effect was not dose-dependent. Data from 3 pooled experiments (N=30 mice per group) showed an engraftment rate of 59% in the saline group, and 47%, 42%, and 21% in the 5mg, 25mg, and 50mg iron dextran groups, respectively, suggesting a dose-dependent effect of iron overload on engraftment (Fig. 1D). In the syngeneic HSCT model, iron overload prior to transplant similarly resulted in delayed RBC and platelet engraftment and decreased rates of overall engraftment (Fig. 1E,F,G).
Conclusions:
Iron overload had a dose-dependent negative effect on overall engraftment after allogeneic and syngeneic HSCT. For allogeneic HSCT, there was also a decrease in overall survival in all the groups receiving iron dextran. For syngeneic HSCT, in which graft rejection cannot occur, iron overload still negatively affected post-transplant engraftment, suggesting that this effect is not due solely to potential immunomodulatory effects of excess iron on graft rejection. The mechanism(s) underlying the effects of iron overload on survival and engraftment rates in allogeneic and syngeneic HSCT, along with the potential benefit of chelation therapy, remain to be determined.
Figure 1: Effect of iron overload on survival and engraftment. Mean ± S.D. for (A) total liver iron and (B) ferritin for allogeneic HSCT. Kaplan-Meier survival estimates for (C) mortality and (D) percent of mice not engrafting >50% of nucleated cells for allogeneic HSCT (N=10 mice per group per replicate; 30 mice total per group). Mean ± S.D. for (E) percent red cell engraftment over time and (F) percent platelet engraftment of time in syngeneic HSCT. Kaplan-Meier survival estimates for (F) percent of mice not engrafting >50% of red blood cells (N=10 mice per group per replicate; 20 mice total per group). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 as compared to the saline control or as specified. Arrow denotes day of transplant.
Spitalnik: New Health Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal